- Belgium comes to Yamashita Park
- Residential Villa in Phuket Entices Remote Workers With Long-Stay Rates
- Rare pieces of French glass art at the Mirai Museum of Art
- Feast on fresh fish and seafood at the 2024 ‘Sakana’ Festival
- Would you like to ride in a Louis Vuitton gondola lift?
- Naked Snow Aquarium
- Festive lights at Yomiuriland will get you feeling the holiday vibes
FDA approves Pfizer vaccine for children in the 5-11 age group
- Tweet
-
- Pin It
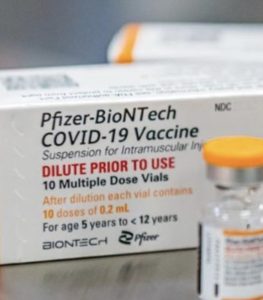
The Food and Drug Administration on Friday authorized Pfizer and BioNTech’s coronavirus vaccine for children five to 11 years old. All that’s needed for the vaccines to reach American children is a CDC approval on Tuesday.
Earlier this week, the US FDA Vaccines and Related Biological Products Advisory Committee voted to recommend the Pfizer-BioNTech coronavirus vaccine for children in the five to 11 age group.
Using the clinical data collected by Pfizer from a trial test involving over 2,200 children, the US drug manufacturer said the vaccine efficacy against COVID-19 was 90.7 percent at least seven days after a second dose. It reported that there were no short-term safety concerns found after the children were vaccinated.
Based on the analysis for the said age group, FDA says the risk of hospitalization is higher than the risk of adverse effects after vaccination. The benefits outweighing the risks led to an overwhelming ‘yes’ vote by the committee members.
Following the results, children in the United States as young as 5 may be eligible to get a shot as soon as next week after final approval by FDA.
Parents in Japan may have to wait longer. Japan requires clinical data to approve the vaccine. Pfizer said it is talking with regulatory authorities in Japan.
About TF Tribe
Related Posts
Latest News
-
Giving your toddler a headstart the Montessori way
According to child development experts, toddlers begin stringing words...
- Posted 5 months ago
- 0
-
FDA approves Pfizer vaccine for children in the 5-11 age group
The Food and Drug Administration on Friday authorized Pfizer and...
- Posted 2 years ago
- 0
-
Toy Story Hotel set to open in Spring 2022 at Tokyo Disney Resort
Artist rendering of the Toy Story Hotel at Tokyo Disney...
- Posted 2 years ago
- 0
-
New dads who take 6 months paternity leave are losers, says private equity investor
Palantir cofounder Joe Lonsdale, an American venture capitalist is under...
- Posted 2 years ago
- 0
-
Japan’s Education Minister Wants Class Size Reduced
Japan’s Education minister Shinsuke Suematsu who assumed his role on...
- Posted 2 years ago
- 0
-
Japan survey finds vaccine immunity level wanes over time in fully vaccinated people especially the elderly
A survey by Soma city in Fukushima reveals the amount...
- Posted 3 years ago
- 0
-
Jon Kent, the son of Superman, is gay in new comics.
The Superboy, Jonathan Kent, son of Clark Kent and Lois...
- Posted 3 years ago
- 0
-
UK Children March to Buckingham Palace to Deliver Petition
On Saturday, TV presenter and naturalist Chris Packham led 100...
- Posted 3 years ago
- 0
-
Michelin-starred Sushi train restaurant opens in Omotesando
Ginza Onodera, a Michelin-starred restaurant known for sushi and tempura...
- Posted 3 years ago
- 0
-
The Strings Omotesando is taking orders for surprise cakes.
The Strings Omotesando is gearing up for the Christmas cake...
- Posted 3 years ago
- 0
-
Facebook puts Instagram Kids project on pause amid mental health concerns
The head of Instagram Adam Mosseri said Monday it will...
- Posted 3 years ago
- 0
-
Emergency status extended in Tokyo and 18 prefectures
Japan extends emergency status over the novel coronavirus pandemic in...
- Posted 3 years ago
- 0
-
Doctors warn: Milk Crate TikTok Challenge Can Cause Terrible Injury
If you have children at home, you probably have heard...
- Posted 3 years ago
- 0
-
Meet the Teenpreneurs who just sold their first startup.
Two teen coders from Seattle, Sage Khanuja, 17, and Nikolas...
- Posted 3 years ago
- 0
-
The important life skills children learn in a Karate class.
Karate is in itself both an art form and sport....
- Posted 3 years ago
- 0
-
Skin irritation from prolonged mask wearing? Here’s a fix.
While it it important to continue wearing a mask for...
- Posted 3 years ago
- 0
-
A Giant Digital Art Garden is Happening at the Tokyo Midtown
Families may not be able to watch the Tokyo...
- Posted 3 years ago
- 0
-
Feeling Nearly Normal
For the first time in 15 months, I hugged a...
- Posted 3 years ago
- 0
-
The Vegan Ramen here is delicious.
As many ramen restaurants in Japan are discovering since the...
- Posted 3 years ago
- 0
-
Steamed Chicken salad with baby potatoes and lettuce
Using a simple olive oil, roasted garlic and lemon instead...
- Posted 3 years ago
- 0
-
Anna Umemiya misses the greatest dad in the world.
Although the life of model, tv personality and product endorser...
- Posted 3 years ago
- 0
-
Study reveals Vegetarians and Pescatarians are less likely to get seriously ill from covid than meat eaters
A new case study, published in the BMJ Nutrition, Prevention...
- Posted 3 years ago
- 0
-
Why I think Tokyo Disneyland attracts more adults than kids.
While being one of the most profitable imports from the...
- Posted 3 years ago
- 0
-
When is the right time to go back to work after having a baby?
Having a baby is exciting, exhausting, and expensive. At some...
- Posted 3 years ago
- 0
-
Japan to stop giving child benefit to high-Income families
Currently, Japan pays a ¥5,000 child allowance as benefit...
- Posted 3 years ago
- 0
-
Life is too short to miss out on these tantalizing desserts.
It’s no understatement to say that not only do the...
- Posted 3 years ago
- 0
-
Japan’s amended Paternal leave may start in October 2022
When Japanese environment minister, Shinjiro Koizumi announced that he was...
- Posted 3 years ago
- 0
-
Japan’s Immigration Bureau warns foreigners about impostor scammers posing as immigration officials
Impostor scams can cost you money, cause identity theft and...
- Posted 3 years ago
- 0
-
Registration for entrance examinations to national and public universities nationwide begins today.
A total of 98,896 students aiming to be admitted to...
- Posted 3 years ago
- 0
-
The most expensive steak sandwich in Japan
Are you ready to spend money on the most expensive...
- Posted 3 years ago
- 0
-
Top 10 Kitchen Essentials People Are Buying in Japan
As the pandemic sway more families into eating at home,...
- Posted 3 years ago
- 0
-
Mom in California dies from Covid after giving birth
Vanessa Cardenas Gonzalez, a 33-year old mom in California died...
- Posted 3 years ago
- 0
-
Keisei Skyliner reserves front car of train for travelers
The Keisei Electric Railway that operates the Keisei Limited Express...
- Posted 3 years ago
- 0
-
“Don’t undercook your meat,” reminds Japan’s health authorities.
With the feasting season upon us, many families will be...
- Posted 3 years ago
- 0
-
How to make a chunky, delicious Clam Chowder kids love.
It’s officially winter. ‘Tis the season for soup –...
- Posted 3 years ago
- 0
-
Why UK Scientists fear New COVID-19 mutant virus may easily infect children.
Up until now, researchers believed children under the age of...
- Posted 3 years ago
- 0
-
Japan tightens restrictions on passengers entering the country from the U.K.
Responding to the threat posed by the highly contagious new...
- Posted 3 years ago
- 0
-
Japan’s recent case count increases slowly but consistently.
According to this week’s COVID update by the World Health...
- Posted 3 years ago
- 0
-
Romain Vandendorpe breaks world record for immersing himself in ice cubes for the longest time.
On December 19, 2020, a 34-year old Frenchman Romain Vandendorpe...
- Posted 3 years ago
- 0
-
WHO Health for All Film Festival invites film-makers to join the film competition
The WHO Health for All Film Festival invites independent film-makers, production companies,...
- Posted 3 years ago
- 0
-
Fuji-Q Highland’s new observation deck to give visitors a premium view of Mount Fuji
The roller coaster at Fuji Q Highland will be closed...
- Posted 3 years ago
- 0
-
Six million light bulbs shine on Sagamiko Pleasure Forest this season
Japan’s largest illumination event, the Sagamiko Pleasure Forest illumination has...
- Posted 3 years ago
- 0
-
2020 Open Innovation Lounge hosted by Hyundai includes Finland-based startup
FLEXOUND Augmented Audio™ was invited to Hyundai Motor Group’s 2020...
- Posted 3 years ago
- 0
-
How to make a French style mocha eclair at home.
Although the pandemic has forced families to keep holiday celebrations...
- Posted 3 years ago
- 0
-
Where to watch dancing sardines in Japan.
Hakkejima Sea Paradise in Yokohama is bringing in “Super Iwashirushon,”...
- Posted 3 years ago
- 0
-
Christmas lights go on display in Ginza
Tokyo is always at the forefront of urban illumination and...
- Posted 3 years ago
- 0
-
Children exposed to violence and abuse likely to age faster, new Harvard study reveals.
Recently published findings (Psychology Bulletin) by researchers at Harvard University...
- Posted 4 years ago
- 0
-
When babies won’t sleep.
Wide-awake-at-night babies drive first-time parents nuts. The reality of having...
- Posted 4 years ago
- 0
-
Japan’s Kanagawa prefecture’s coronavirus test is significantly quicker..
WHO-endorsed Nucleic Acid Amplification Tests (NAAT), a method that detects...
- Posted 4 years ago
- 0
-
Japanese life hack products every resident should know.
The kitchen is one of the dirtiest areas of the...
- Posted 4 years ago
- 0
-
Harry Potter and the Cursed Child play on Tokyo stage in 2022.
People in Tokyo will have every reason to get excited...
- Posted 4 years ago
- 0
-
Perfect Winter Getaway: Nagano and Karuizawa
Families looking for a holiday retreat away from the deafening...
- Posted 4 years ago
- 0
-
Forest-style learning: Komazawa Park International School
The school’s increasingly popular forest-style learning is a beacon of...
- Posted 4 years ago
- 0
-
Tokyo’s 3-day Flea Market to showcase vintage items and antiques from all over Japan.
If you want to snag a bargain for vintage decor...
- Posted 2 days ago
- 0
-
Osaka Science Museum gets a makeover after 35 years
The Osaka Science Museum (formerly called Osaka Electrical Science Museum)...
- Posted 4 days ago
- 0
-
Hayao Miyazaki one of Time’s most influential people of 2024
Japanese anime director Hayao Miyazaki is among Time’s 100 most...
- Posted 1 week ago
- 0
-
Luxurious Resort Opens Exquisite Old-World Bar By The Beach
The Anam Mui Ne, an Indochine-era inspired resort in Vietnam’s popular...
- Posted 1 week ago
- 0
-
Where were you on April 8?
Not since Y2K have I experienced such a wave of...
- Posted 1 week ago
- 0
-
A capital adventure in Vietnam
A few decades ago, the nation of Vietnam was synonymous...
- Posted 2 weeks ago
- 0
-
Belgium comes to Yamashita Park
Belgian Beer Weekend, Tokyo’s annual spring beer fest, boasts an...
- Posted 2 weeks ago
- 0
-
Osaka Science Museum gets a makeover after 35 years
The Osaka Science Museum (formerly called Osaka Electrical Science...
- April 23, 2024
- 0
-
Hayao Miyazaki one of Time’s most influential people of 2024
Japanese anime director Hayao Miyazaki is among Time’s 100...
- April 18, 2024
- 0
-
Luxurious Resort Opens Exquisite Old-World Bar By The Beach
The Anam Mui Ne, an Indochine-era inspired resort in Vietnam’s...
- April 17, 2024
- 0
-
Giving your toddler a headstart the Montessori way
According to child development experts, toddlers begin stringing...
- November 30, 2023
- 0
-
FDA approves Pfizer vaccine for children in the 5-11 age group
The Food and Drug Administration on Friday authorized Pfizer...
- October 30, 2021
- 0
-
Toy Story Hotel set to open in Spring 2022 at Tokyo Disney Resort
Artist rendering of the Toy Story Hotel at Tokyo...
- October 29, 2021
- 0
-
New dads who take 6 months paternity leave are losers, says private equity investor
Palantir cofounder Joe Lonsdale, an American venture capitalist is...
- October 29, 2021
- 0
-
Japan’s Education Minister Wants Class Size Reduced
Japan’s Education minister Shinsuke Suematsu who assumed his role...
- October 28, 2021
- 0
-
Japan survey finds vaccine immunity level wanes over time in fully vaccinated people especially the elderly
A survey by Soma city in Fukushima reveals the...
- October 18, 2021
- 0
-
Jon Kent, the son of Superman, is gay in new comics.
The Superboy, Jonathan Kent, son of Clark Kent and...
- October 12, 2021
- 0
-
UK Children March to Buckingham Palace to Deliver Petition
On Saturday, TV presenter and naturalist Chris Packham led...
- October 11, 2021
- 0
-
Michelin-starred Sushi train restaurant opens in Omotesando
Ginza Onodera, a Michelin-starred restaurant known for sushi and...
- October 11, 2021
- 0
-
The Strings Omotesando is taking orders for surprise cakes.
The Strings Omotesando is gearing up for the Christmas...
- October 1, 2021
- 0
-
Facebook puts Instagram Kids project on pause amid mental health concerns
The head of Instagram Adam Mosseri said Monday it...
- September 27, 2021
- 0
-
Emergency status extended in Tokyo and 18 prefectures
Japan extends emergency status over the novel coronavirus pandemic ...
- September 13, 2021
- 0
-
Doctors warn: Milk Crate TikTok Challenge Can Cause Terrible Injury
If you have children at home, you probably have...
- August 27, 2021
- 0
-
Meet the Teenpreneurs who just sold their first startup.
Two teen coders from Seattle, Sage Khanuja, 17, and...
- July 27, 2021
- 0
-
The important life skills children learn in a Karate class.
Karate is in itself both an art form and...
- July 26, 2021
- 0
-
Skin irritation from prolonged mask wearing? Here’s a fix.
While it it important to continue wearing a mask...
- July 21, 2021
- 0
-
A Giant Digital Art Garden is Happening at the Tokyo Midtown
Families may not be able to watch the...
- July 14, 2021
- 0
-
The Vegan Ramen here is delicious.
As many ramen restaurants in Japan are discovering since...
- July 7, 2021
- 0
-
Steamed Chicken salad with baby potatoes and lettuce
Using a simple olive oil, roasted garlic and lemon...
- June 21, 2021
- 0
-
Anna Umemiya misses the greatest dad in the world.
Although the life of model, tv personality and product...
- June 18, 2021
- 0
-
Study reveals Vegetarians and Pescatarians are less likely to get seriously ill from covid than meat eaters
A new case study, published in the BMJ Nutrition,...
- June 9, 2021
- 0
-
Why I think Tokyo Disneyland attracts more adults than kids.
While being one of the most profitable imports from...
- March 2, 2021
- 0
-
When is the right time to go back to work after having a baby?
Having a baby is exciting, exhausting, and expensive. At...
- February 9, 2021
- 0
-
Japan to stop giving child benefit to high-Income families
Currently, Japan pays a ¥5,000 child allowance as...
- February 3, 2021
- 0
-
Life is too short to miss out on these tantalizing desserts.
It’s no understatement to say that not only do...
- February 2, 2021
- 0
-
Japan’s amended Paternal leave may start in October 2022
When Japanese environment minister, Shinjiro Koizumi announced that he...
- January 28, 2021
- 0
-
Japan’s Immigration Bureau warns foreigners about impostor scammers posing as immigration officials
Impostor scams can cost you money, cause identity theft...
- January 26, 2021
- 0
-
Registration for entrance examinations to national and public universities nationwide begins today.
A total of 98,896 students aiming to be admitted...
- January 25, 2021
- 0
-
The most expensive steak sandwich in Japan
Are you ready to spend money on the most...
- January 6, 2021
- 0
-
Top 10 Kitchen Essentials People Are Buying in Japan
As the pandemic sway more families into eating at...
- December 31, 2020
- 0
-
Mom in California dies from Covid after giving birth
Vanessa Cardenas Gonzalez, a 33-year old mom in California...
- December 30, 2020
- 0
-
Keisei Skyliner reserves front car of train for travelers
The Keisei Electric Railway that operates the Keisei Limited...
- December 29, 2020
- 0
-
“Don’t undercook your meat,” reminds Japan’s health authorities.
With the feasting season upon us, many families will...
- December 26, 2020
- 0
-
How to make a chunky, delicious Clam Chowder kids love.
It’s officially winter. ‘Tis the season for soup...
- December 24, 2020
- 0
-
Why UK Scientists fear New COVID-19 mutant virus may easily infect children.
Up until now, researchers believed children under the age...
- December 23, 2020
- 0
-
Japan tightens restrictions on passengers entering the country from the U.K.
Responding to the threat posed by the highly contagious...
- December 23, 2020
- 0
-
Japan’s recent case count increases slowly but consistently.
According to this week’s COVID update by the World...
- December 23, 2020
- 0
-
Romain Vandendorpe breaks world record for immersing himself in ice cubes for the longest time.
On December 19, 2020, a 34-year old Frenchman Romain...
- December 22, 2020
- 0
-
WHO Health for All Film Festival invites film-makers to join the film competition
The WHO Health for All Film Festival invites independent film-makers, production...
- December 21, 2020
- 0
-
Fuji-Q Highland’s new observation deck to give visitors a premium view of Mount Fuji
The roller coaster at Fuji Q Highland will be...
- December 20, 2020
- 0
-
Six million light bulbs shine on Sagamiko Pleasure Forest this season
Japan’s largest illumination event, the Sagamiko Pleasure Forest illumination...
- December 15, 2020
- 0
-
2020 Open Innovation Lounge hosted by Hyundai includes Finland-based startup
FLEXOUND Augmented Audio™ was invited to Hyundai Motor Group’s...
- December 9, 2020
- 0
-
How to make a French style mocha eclair at home.
Although the pandemic has forced families to keep holiday...
- December 7, 2020
- 0
-
Where to watch dancing sardines in Japan.
Hakkejima Sea Paradise in Yokohama is bringing in “Super...
- November 12, 2020
- 0
-
Christmas lights go on display in Ginza
Tokyo is always at the forefront of urban illumination...
- November 11, 2020
- 0
-
Children exposed to violence and abuse likely to age faster, new Harvard study reveals.
Recently published findings (Psychology Bulletin) by researchers at Harvard...
- August 13, 2020
- 0
-
When babies won’t sleep.
Wide-awake-at-night babies drive first-time parents nuts. The reality of...
- July 10, 2020
- 0
-
Japan’s Kanagawa prefecture’s coronavirus test is significantly quicker..
WHO-endorsed Nucleic Acid Amplification Tests (NAAT), a method that...
- July 7, 2020
- 0
-
Japanese life hack products every resident should know.
The kitchen is one of the dirtiest areas of...
- July 2, 2020
- 0
-
Harry Potter and the Cursed Child play on Tokyo stage in 2022.
People in Tokyo will have every reason to get...
- February 14, 2020
- 0
-
Perfect Winter Getaway: Nagano and Karuizawa
Families looking for a holiday retreat away from the...
- February 14, 2020
- 0
-
Forest-style learning: Komazawa Park International School
The school’s increasingly popular forest-style learning is a beacon...
- February 13, 2020
- 0
-
Chinese garlic is cheap but I won’t be buying it ever again. Here’s why.
We know that when it comes to food, the...
- August 22, 2016
- 1
-
If you have this line on your palm, you have what it takes to be wealthy and famous.
You know you’ve walked past “Te saw” or palm...
- May 12, 2016
- 1
-
Tokyo’s 3-day Flea Market to showcase vintage items and antiques from all over Japan.
If you want to snag a bargain for vintage...
- April 25, 2024
- 0










